
Chemical Kinetics Class 12 Notes PDF | Handwritten & Easy to Understand
Are you looking for clear, concise, and exam-focused handwritten notes for Chemical Kinetics Class 12? You’ve come to the right place! In this PDF, we provide easy-to-understand handwritten notes that cover all important concepts, formulas, and derivations from the Class 12 Chemistry NCERT syllabus. Whether you’re preparing for CBSE board exams, NEET, or JEE, these notes are perfect for quick revision and last-minute preparation. Download now and strengthen your understanding of rate of reaction, order, molecularity, integrated rate equations, and more — all in a student-friendly format
Chemical Kinetics Class 12 Notes PDF
Chemical kinetics short notes
1. Rate of Reaction
Average Rate:
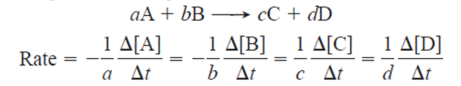
Instantaneous Rate

2. Rate Law (Rate Equation)

3.Units of Rate Constant (k)

4. Order of Reaction
- The sum of powers of concentration terms in rate law.
- Can be 0, 1, 2, or fractional.
- Determined experimentally.
5. Molecularity
- No. of molecules colliding in a single-step reaction.
- Always a whole number.
- Not applicable to complex (multi-step) reactions
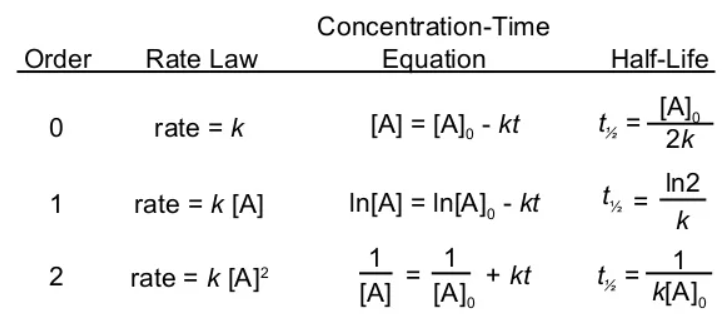
6. Integrated Rate Equations
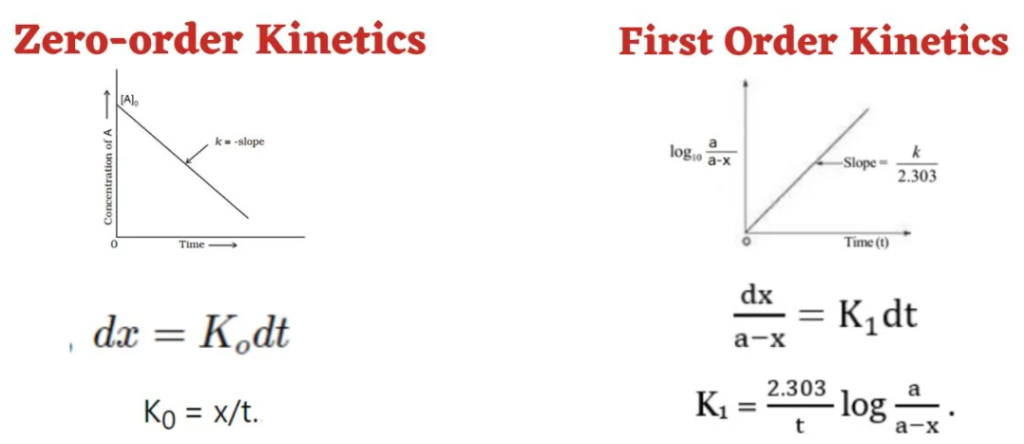
7.Half-life
8.Arrhenius Equation

Graph


