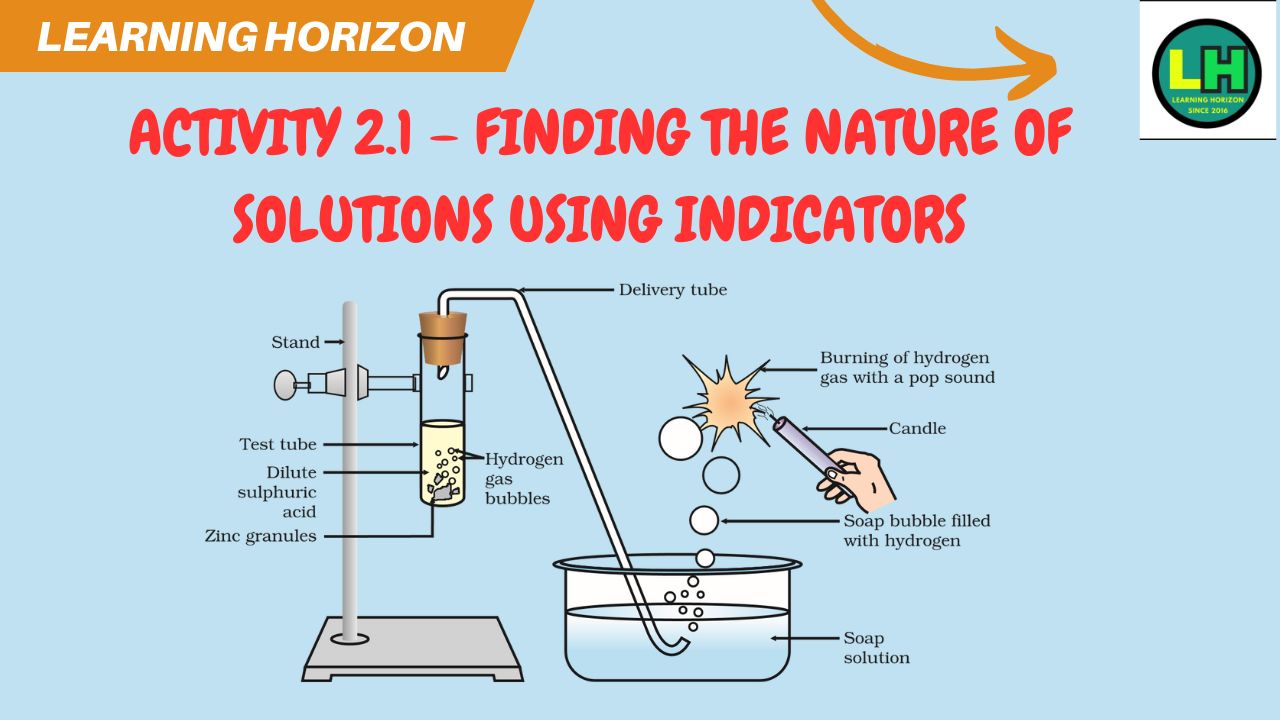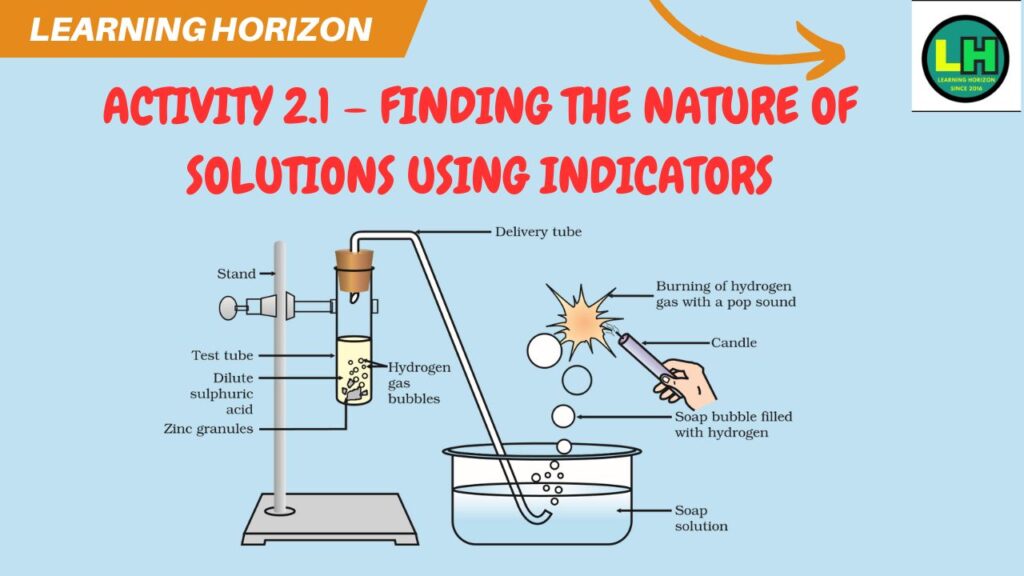
Activity 2.1 – Finding the nature of solutions using indicators
Activity 2.1 – Finding the nature of solutions using indicators
Objective:
To test the nature (acidic, basic or neutral) of various substances using different indicators.
Materials Required:
- Test tubes
- Dropper
- Solutions of:
- Lemon juice
- Vinegar
- Baking soda solution
- Soap solution
- Indicators:
- Litmus paper (red and blue)
- Phenolphthalein
- Methyl orange
Procedure:
- Take small amounts of each solution in separate test tubes.
- Test each solution with:
- Red and blue litmus paper
- Phenolphthalein solution
- Methyl orange solution
Activity 2.1 – Finding the nature of solutions using indicators
Observations:
| Substance | Red Litmus | Blue Litmus | Phenolphthalein | Methyl Orange | Nature of Solution |
|---|---|---|---|---|---|
| Lemon juice | No change | Turns red | Colourless | Red | Acidic |
| Vinegar | No change | Turns red | Colourless | Red | Acidic |
| Baking soda solution | Turns blue | No change | Pink | Yellow | Basic |
| Soap solution | Turns blue | No change | Pink | Yellow | Basic |
Conclusion:
- Acidic substances turn blue litmus red, and keep red litmus unchanged.
- Basic substances turn red litmus blue, and keep blue litmus unchanged.
- Phenolphthalein gives pink color in bases and remains colorless in acids.
- Methyl orange turns red in acids and yellow in bases.
Frequently ask Question answer
Q1. What is the aim of Activity 2.1?
Answer:
To test the nature of different solutions (acidic or basic) using various indicators like litmus, phenolphthalein, and methyl orange.
Q2. What are the different indicators used in this activity?
Answer:
The indicators used are:
- Red and blue litmus paper
- Phenolphthalein
- Methyl orange
Q3. What happens when blue litmus paper is dipped in lemon juice?
Answer:
Blue litmus paper turns red, showing that lemon juice is acidic.
Q4. What change is observed when red litmus paper is dipped in soap solution?
Answer:
Red litmus paper turns blue, showing that soap solution is basic.
Q5. What color does phenolphthalein show in an acidic and basic solution?
Answer:
- In acidic solution → Colorless
- In basic solution → Pink
Q6. What color does methyl orange show in acids and bases?
Answer:
- In acidic solution → Red
- In basic solution → Yellow
Q7. Which substances tested in this activity are acidic in nature?
Answer:
- Lemon juice
- Vinegar
Q8. Which substances tested are basic in nature?
Answer:
- Baking soda solution
- Soap solution
Q9. Why are indicators used in this activity?
Answer:
Indicators are used to identify whether a substance is acidic or basic by showing a visible color change.
Q10. What conclusion do you draw from Activity 2.1?
Answer:
Acids and bases can be distinguished using indicators.
- Acids turn blue litmus red.
- Bases turn red litmus blue.
- Different indicators show characteristic color changes in acidic and basic media.
Activity 2.1 – Finding the nature of solutions using indicators
READ ALSO: burning of magnesium ribbon