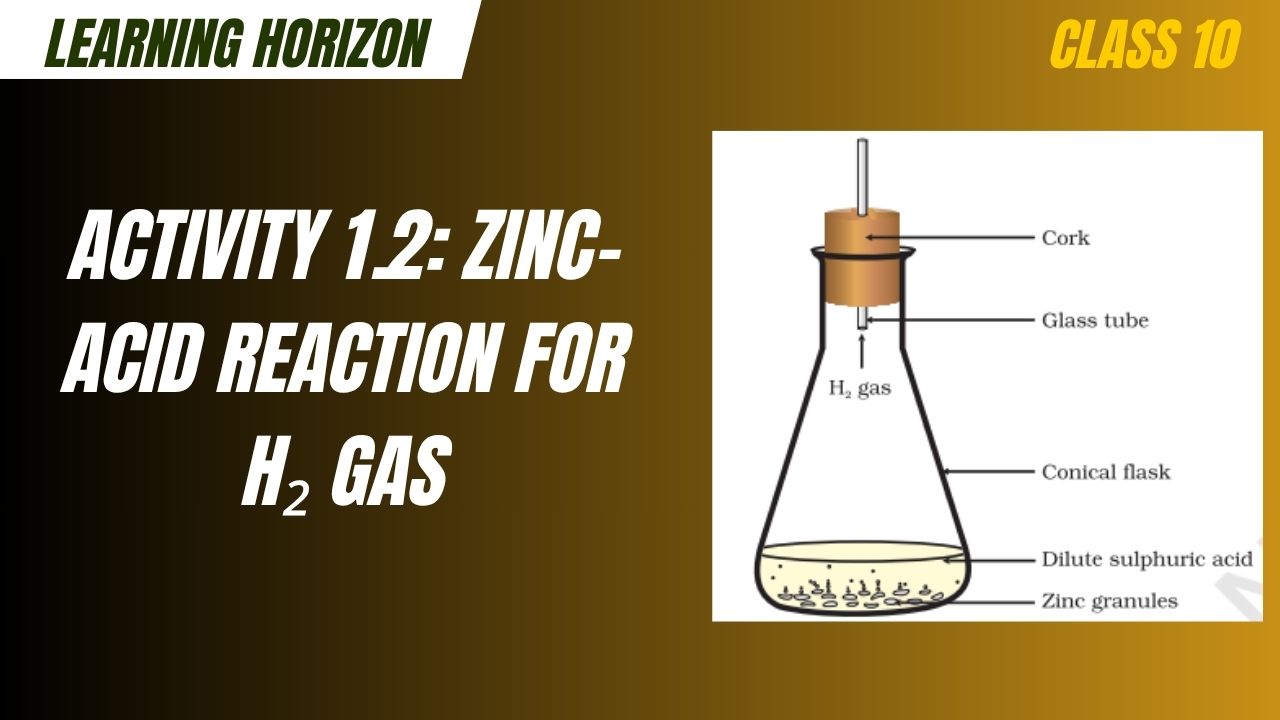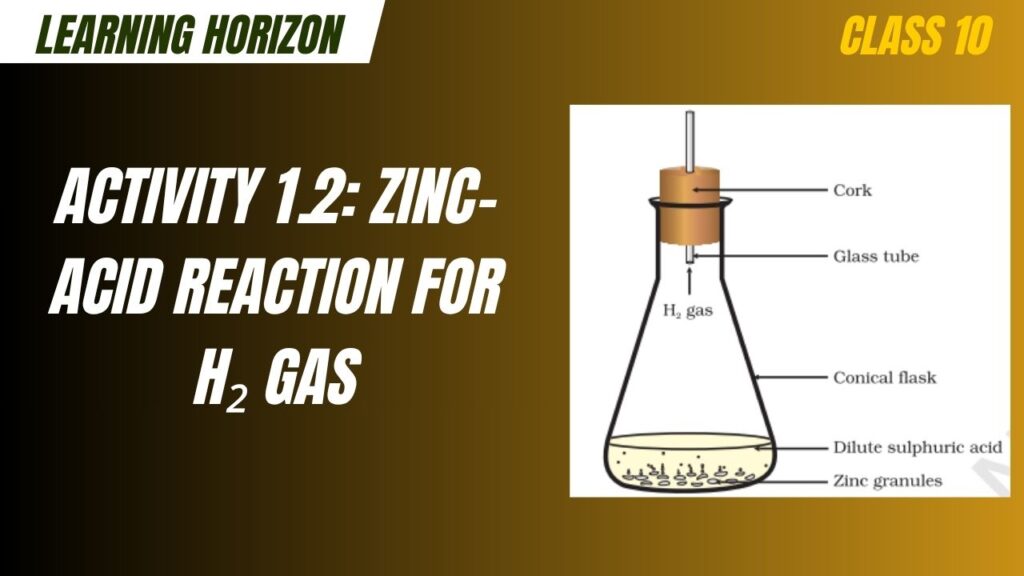
Activity 1.2: Zinc-Acid Reaction for H₂ Gas
Activity 1.2: Zinc-Acid Reaction for H₂ Gas is a fundamental science experiment covered in Class 10 Science that demonstrates the chemical reaction between a metal (zinc) and a dilute acid (sulphuric acid). This activity highlights the displacement of hydrogen from dilute sulphuric acid by zinc, resulting in the evolution of hydrogen gas. It helps students understand metal reactivity, single displacement reactions, and gas evolution processes in chemistry. The experiment also introduces the concept of the reactivity series and safe lab practices in handling acids and reactive metals.
chemical reaction and equation class 10
Chemical Reaction Involved:

When zinc (Zn) reacts with dilute sulphuric acid (H₂SO₄), hydrogen gas (H₂) is released, and zinc sulphate (ZnSO₄) is formed as a salt.
Balanced Chemical Equation: Zn (s)+H2SO4 (aq)→ZnSO4 (aq)+H2 (g)
Type of Reaction:
This is a single displacement reaction, where zinc, a more reactive metal, displaces hydrogen from sulphuric acid.
Explanation of the Process:
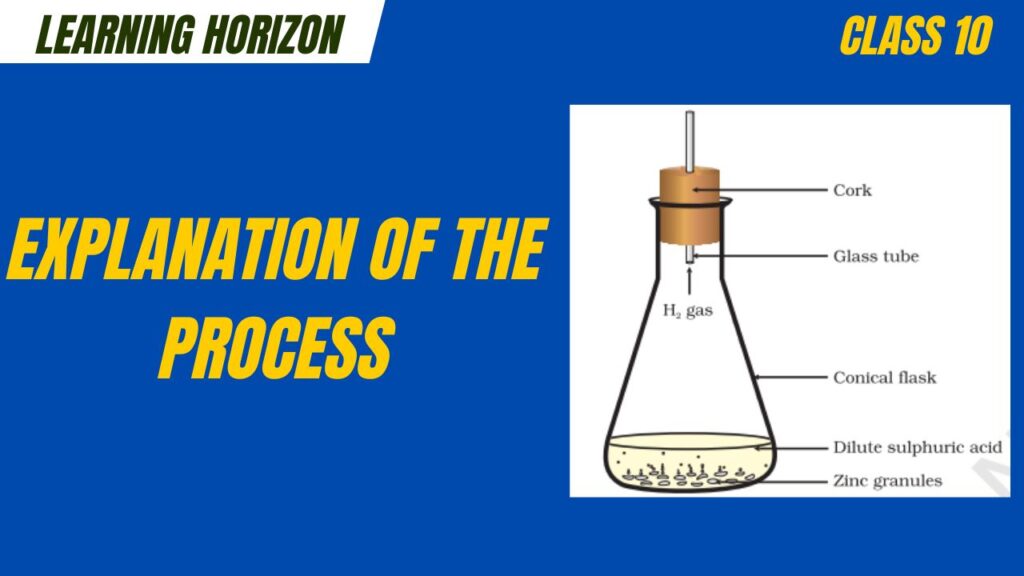
- Zinc Reactivity:
- Zinc is a moderately reactive metal.
- It lies above hydrogen in the reactivity series.
- Hence, it can displace hydrogen from acids.
- Action of Sulphuric Acid:
- Dilute sulphuric acid contains free hydrogen ions (H⁺) in solution.
- When zinc is added to dilute H₂SO₄, it donates electrons to the hydrogen ions.
- Electron Transfer (Redox Reaction):
- Zinc loses two electrons to form Zn²⁺ ions (oxidation).
- Hydrogen ions gain electrons and form hydrogen gas (reduction).
- Formation of Products:
- The Zn²⁺ ions combine with the SO₄²⁻ ions in the acid to form zinc sulphate (ZnSO₄).
- The hydrogen gas (H₂) bubbles out of the solution.
Observations:

- Effervescence (bubbling) occurs due to the release of hydrogen gas.
- The zinc metal slowly dissolves in the acid.
- A colorless solution of zinc sulphate remains.
Test for Hydrogen Gas:
- Bring a burning splint near the mouth of the test tube.
- Hydrogen burns with a ‘pop’ sound, confirming its presence.
Safety Precautions:
- Conduct the experiment in a well-ventilated area or fume hood.
- Handle sulphuric acid with care – it is corrosive.
- Wear gloves and safety goggles.
Activity 1.2: Zinc-Acid Reaction for H₂ Gas
Viva Questions and Answers
- Q1: What is the aim of Activity 1.2?
A: The aim is to study the reaction of zinc with dilute sulphuric acid and observe the evolution of hydrogen gas.
- Q2: What type of reaction takes place between zinc and dilute sulphuric acid?
A: It is a single displacement reaction and also a redox reaction.
- Q3: Write the balanced chemical equation for the reaction.
A: Zn (s)+H2SO4 (aq)→ZnSO4 (aq)+H2 (g
- Q4: What gas is evolved during the reaction? How do you test it?
A: Hydrogen gas is evolved. It is tested by bringing a burning splint near the mouth of the test tube; it burns with a ‘pop’ sound.
- Q5: Why does zinc react with sulphuric acid but copper does not?
A: Zinc is more reactive than hydrogen (above hydrogen in the reactivity series), so it displaces hydrogen from the acid. Copper is less reactive and cannot displace hydrogen.
- Q6: What is observed when zinc reacts with dilute sulphuric acid?
A: Effervescence or bubbling is observed due to the release of hydrogen gas, and zinc gradually dissolves.
- Q7: Which compound is formed in the solution after the reaction?
A: Zinc sulphate (ZnSO₄) is formed.
- Q8: Why is dilute acid used and not concentrated acid in this activity?
A: Dilute acid is safer and reacts controllably with zinc. Concentrated acid is corrosive and dangerous for classroom use.
- Q9: What is the role of sulphuric acid in the reaction?
A: It provides hydrogen ions (H⁺) that get reduced to form hydrogen gas.
- Q10: Is this a physical or chemical change? Why?
A: It is a chemical change because new substances (hydrogen gas and zinc sulphate) are formed, and it is irreversible.
Read also : Activity 1.1 : burning of magnesium ribbon