Table of Contents
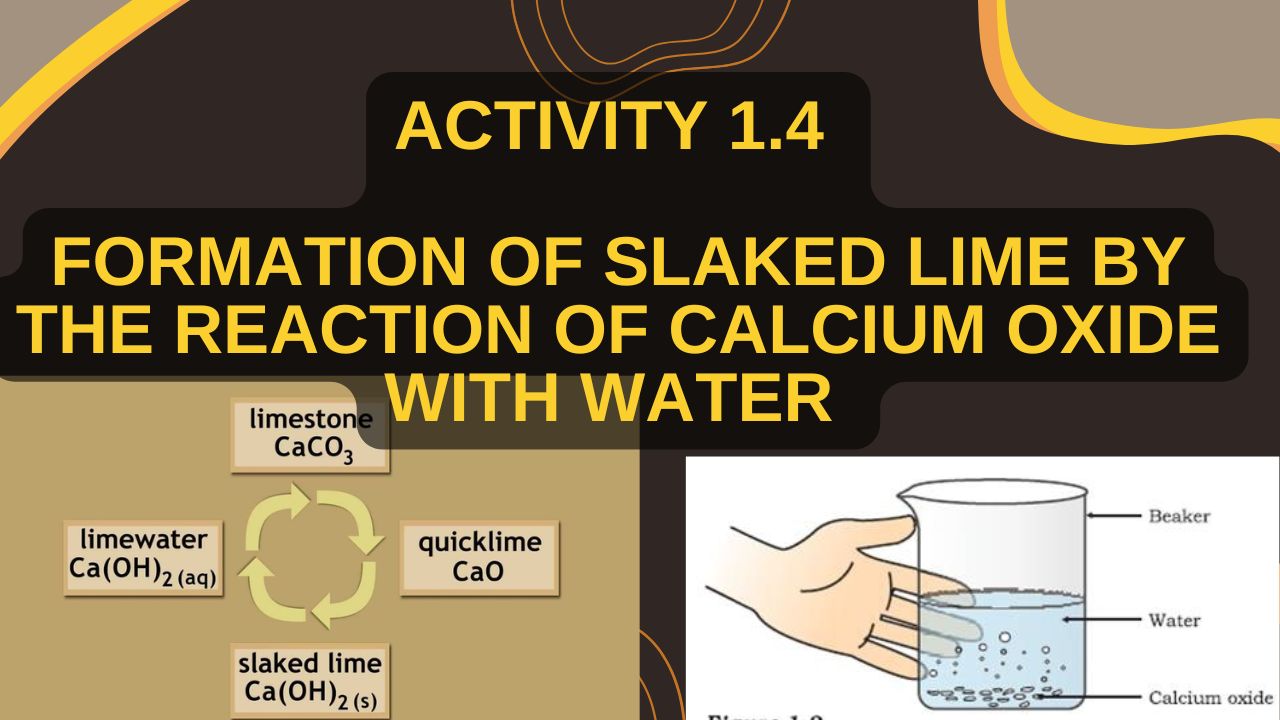
Activity 1.4 -Formation of slaked lime by the reaction of calcium oxide with water
Aim:
To observe the exothermic reaction between calcium oxide (quick lime) and water, resulting in the formation of slaked lime
Materials Required:
- Calcium oxide (CaO)
- Water (H₂O)
- Beaker
- Glass rod
Activity 1.4 -Formation of slaked lime by the reaction of calcium oxide with water
Materials Required
- Take a small amount of quick lime (CaO) in a beaker.
- Slowly add water to it.
- Stir the mixture with a glass rod.
- Observe the changes in temperature and appearance.
Observation:
- A hissing sound is produced.
- The beaker becomes hot (exothermic reaction).
- A white, powdery substance (slaked lime) is formed.
Chemical Reaction:
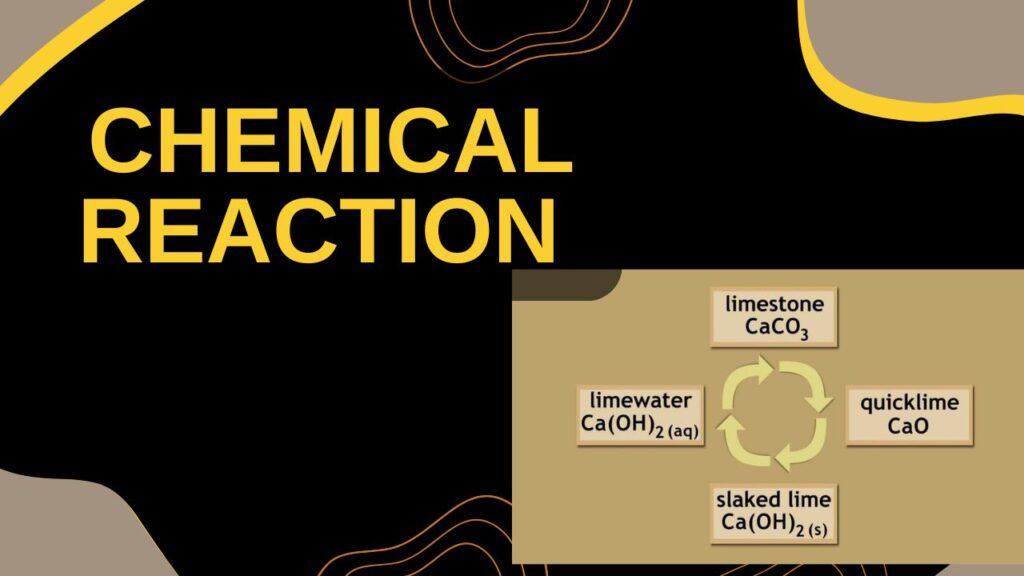
CaO (s) + H₂O (l) → Ca(OH)₂ (aq)+heat
Conclusion:
- Calcium oxide reacts vigorously with water to form calcium hydroxide (slaked lime).
- The reaction is exothermic as it releases heat.
Real-Life Application:
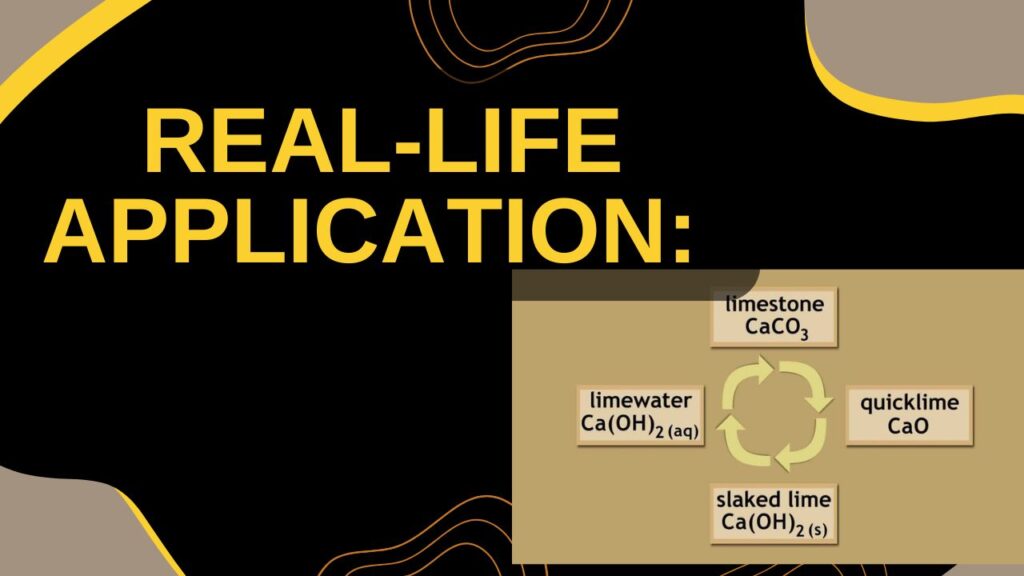
- Slaked lime is used in whitewashing walls.
- It is also used in treating acidic soil in agriculture.
- Activity 1.4 -Formation of slaked lime by the reaction of calcium oxide with water
Resource : NCERT
Read more :
| Activity | 1.1 |
| Activity | 1.2 |
| Activity | 1.5 |